Small spheres save enzymes for biocatalysis
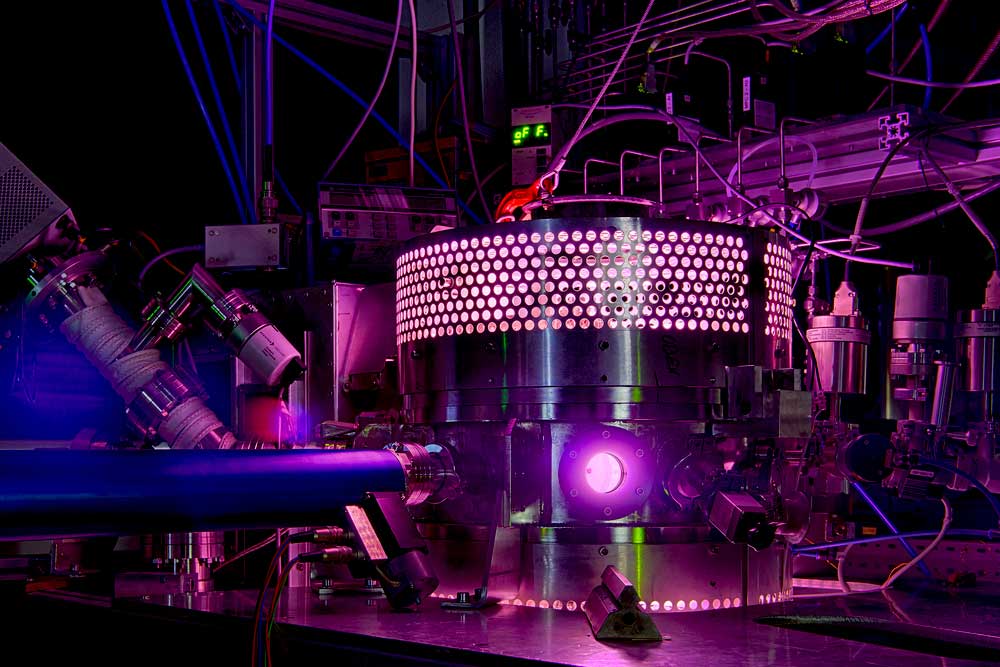
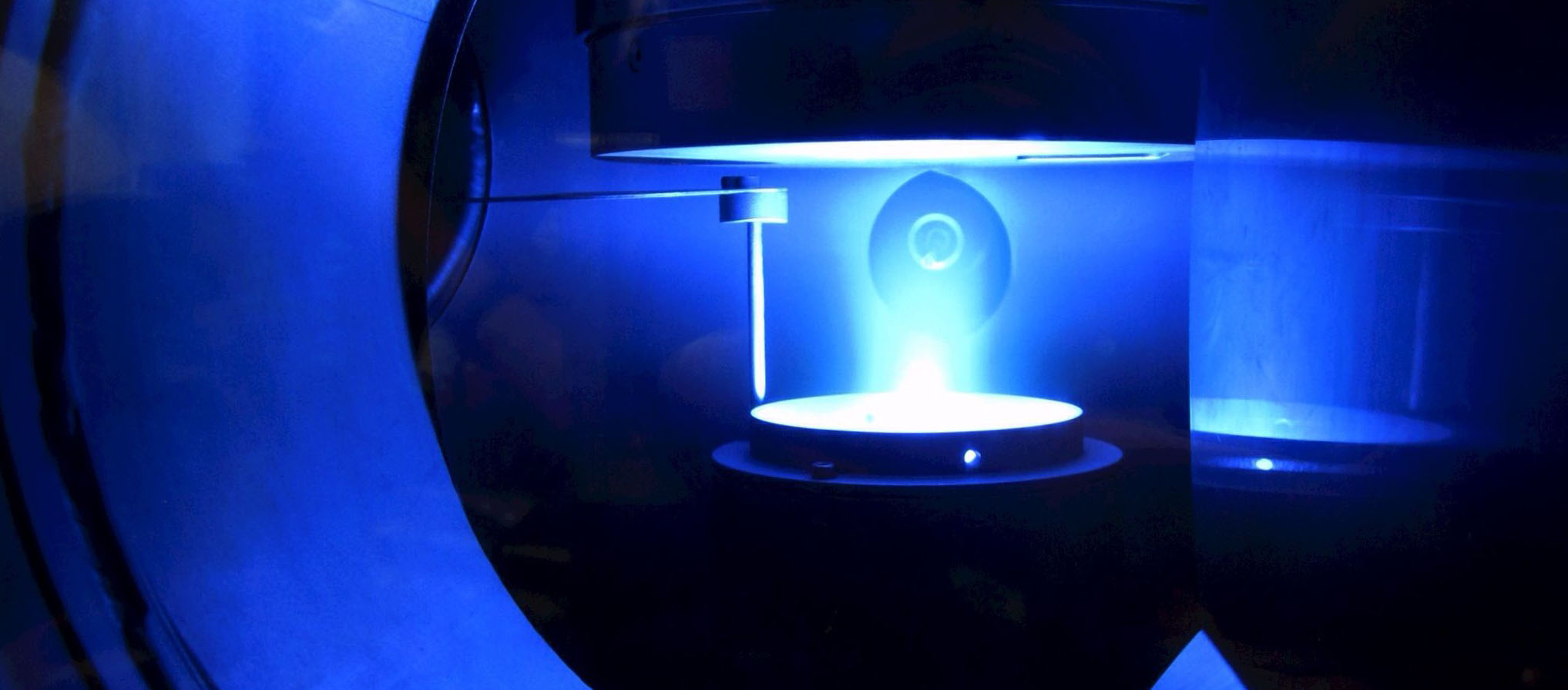
Plasmas can supply the co-substrate for the biocatalysis of valuable substances, but pity the enzymes. If the latter are attached to small spheres, they work protected and up to 44 times longer.
Some enzymes, such as the one from fungi studied here, are able to produce valuable substances such as the fragrance (R)-1-phenylethanol. To do this, they convert a less expensive substrate using a cosubstrate. A research team from the Department of Biology at Ruhr-Universität Bochum came up with the idea of supplying them with this cosubstrate via a plasma - a crazy idea, as plasmas generally have a destructive effect on biomolecules. However, using several tricks, the researchers led by Prof. Dr. Julia Bandow and Dr. Tim Dirks succeeded. They have now refined one of these tricks and thus improved the process: they attach the enzymes to small balls to hold them to the bottom of the reactor and like this protect them from the harmful influence of the plasma. By choosing the most suitable type of ball, they were able to increase the stability of the enzyme 44-fold. They report in the Journal of the Royal Society Interface from October 25, 2023.
Model enzyme from an edible mushroom
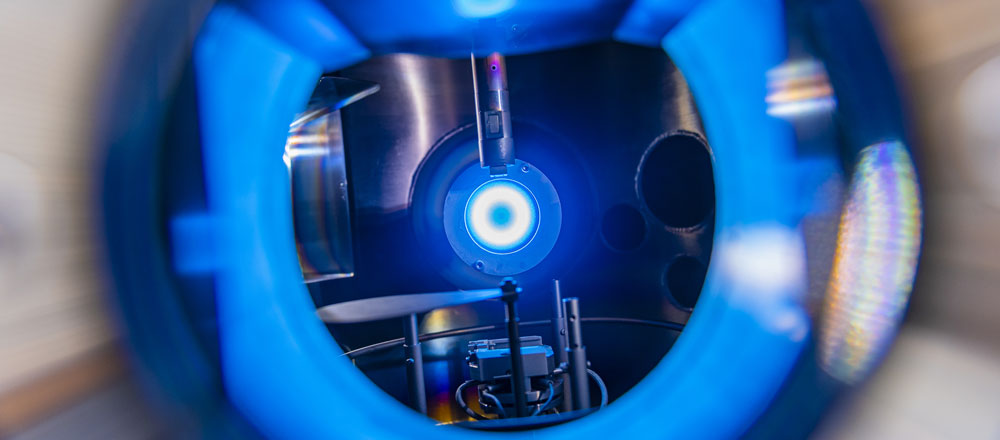
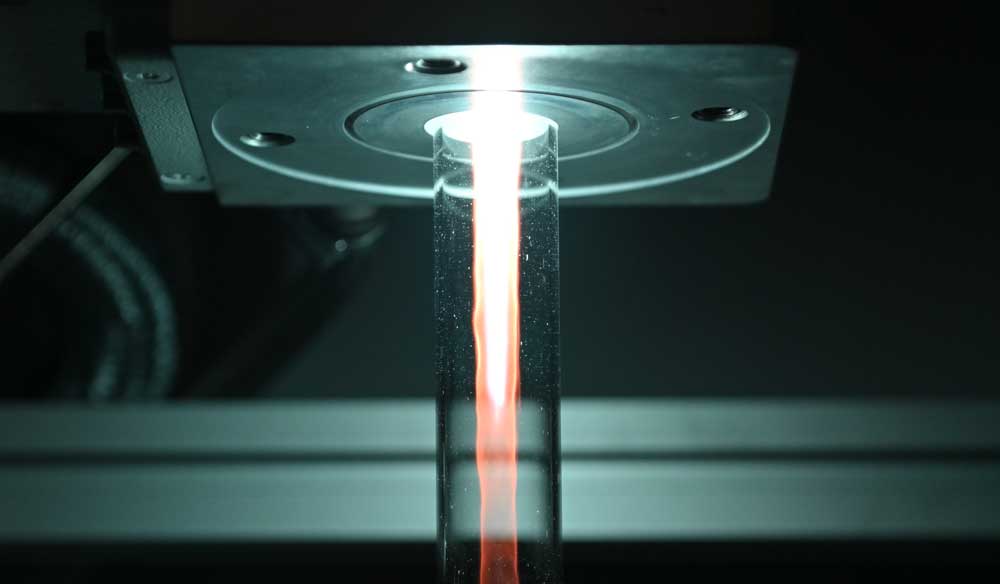
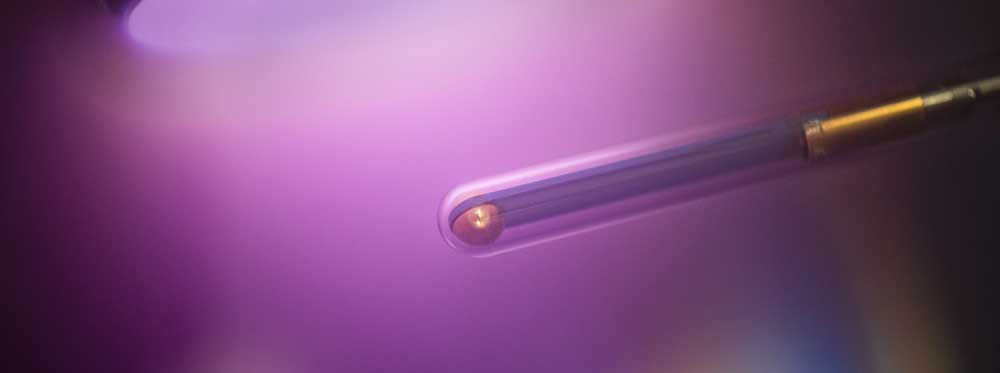
"In plasma-driven biocatalysis, we want to operate enzymes that use hydrogen peroxide to convert a substrate into a more valuable product using technical plasmas," explains Julia Bandow, Head of the Chair of Applied Microbiology. The plasmas - energetically charged gases - produce hydrogen peroxide as well as a variety of reactive species.

The researchers use the non-specific peroxigenase (AaeUPO) from the edible fungus Agrocybe aegerita as a model enzyme. In initial studies, they were able to show that although plasma-driven biocatalysis works with it, there are also key limitations. "The decisive factor was that the enzymes react sensitively to the plasma treatment and are therefore inactivated within a short period of time," explains Tim Dirks, first author of the current study. "To prevent this, we use the method of enzyme immobilization, i.e. attaching the enzymes to so-called beads: small spheres with a porous surface."
Spheres keep the enzymes at the bottom
Due to gravity, these spheres lie on the bottom of the sample and provide a protective zone between the plasma phase at the top and the enzymes. The research team observed early on that the choice of different immobilization methods also led to different survival rates of enzymes. The aim of the current study was therefore to investigate the effect of different immobilization methods on the plasma stability of enzymes using a larger selection of enzymes.
Five different enzymes were selected, two of which also convert hydrogen peroxide and three of which do not require hydrogen peroxide for their activity. The researchers tested nine different types of beads, some of which had a resin surface and others a silica surface with or without a polymer coating. After immobilization, the enzymes were treated with plasma for up to five minutes. The researchers then compared their residual activity with untreated controls.
The path to new applications
The beads with resin surfaces showed the best results for all five enzymes. "The amino and epoxy-butyl beads performed best," like these," says Tim Dirks. In both cases, the enzymes form a strong, covalent bond with the carrier material, which cannot be dissolved. "This type of immobilization appears to limit the mobility of the enzymes, which makes them less susceptible to plasma-induced inactivation," concludes Tim Dirks. The team extended the plasma treatment times for the most promising candidates to up to one hour and, like this, was able to increase the stability of the enzymes under plasma treatment by up to a factor of 44 through immobilization. "The findings of this study thus pave the way for new applications that aim to combine enzymes with technical plasmas in the future," the researchers like this.
adapted from Maike Drießen, RUB